
Sai Batchu1, Michael Joseph Diaz2, Lauren Ladehoff3, Kevin Root2, Brandon Lucke-Wold4*
1Montville, NJ, United States
2College of Medicine, University of Florida, Gainesville, FL
3Morsani College of Medicine, University of South Florida, Tampa, FL
4Department of Neurosurgery, University of Florida, Gainesville, FL
*Corresponding Author: Brandon Lucke-Wold, Department of Neurosurgery, University of Florida, Gainesville, FL.
Received Date: September 28, 2022
Accepted Date: October 19, 2022
Published Date: June 24, 2023
Citation: Sai Batchu, Michael Joseph Diaz, Lauren Ladehoff, Kevin Root, Brandon Lucke-Wold. (2023). “Differential Immune Cell Recruitment Between Ruptured and Unruptured Intracranial Aneurysms”. Clinical Psychology and Mental Health Care, 5(1); DOI: http;//doi.org/06.2023/1.10069.
Copyright: © 2023 Brandon Lucke-Wold. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background: Accumulating evidence implicates infiltrating inflammatory cells in intracranial aneurysms. However, few studies have examined immunological differences between aneurysm rupture statuses. To address this gap, the present study aimed to infer the proportions of specific immune cell types in ruptured and unruptured intracranial aneurysms.
Methods: An established machine-learning deconvolution algorithm was applied on RNA-sequencing data developed from vessel wall tissue of 21 ruptured and 21 unruptured intracranial aneurysms. A validated gene signature matrix of human hematopoietic cell subsets was used to infer the relative fractions of immune cells present within the original aneurysm tissues.
Results: Deconvolution of the bulk gene expression data showed significantly increased plasma cells, CD8+ T cells, and activated natural killer cells in unruptured aneurysms compared to ruptured aneurysms.
Conclusion: Specific lymphoid elements may be involved in an inflammatory reaction prior to intracranial aneurysm rupture.
Introduction:
Intracranial aneurysms (IA) are common cerebrovascular abnormalities prevalent in about 3.2% of the general population [1]. Ruptured aneurysms present with lethality in up to 65% of cases and can significantly disable those who survive [2-3]. Current preventive treatments of unruptured IA carry notable risks of complications [4]. Novel strategies to inhibit the rupture of IA are therefore warranted. Despite several risk factors identified for IA, no safe noninvasive therapies have been established as of yet partly due to the lack of knowledge of mechanisms leading to the rupture of IA. However, recent evidence has implicated the role of inflammation in aneurysm pathogenesis.
The common pathway for aneurysm formation begins with hemodynamic stress causing endothelial injury and dysfunction. The resulting inflammatory response in the vessel wall leads to internal elastic lamina disruption and extracellular matrix remodeling, presumably causing the formation of an aneurysm. From here, further vessel wall degeneration ultimately leads to IA rupture [5]. The transition from unruptured IA to the ruptured phenotype has been associated with matrix metalloproteinases, suggesting breakdown of vessel extracellular matrices lead to rupture [6]. Metalloproteinases are produced abundantly by leukocytes [7-8]. Indeed, histological analyses have reported infiltration of T cells, B cells, and macrophages in aneurysm walls [9-10]. Moreover, complement proteins and immunoglobulins have also been found increased in ruptured compared to unruptured IA, suggesting a humoral response and B lymphocyte contribution to rupture pathogenesis [11]. Taken together, these findings suggest differential immune involvement between aneurysm rupture statuses. However, the exact immune cell subtypes involved still remain obscure.
Immunohistochemistry and flow cytometry are common techniques to analyze cell compositions of tissues but have notable limitations. Unambiguous classifications of cell types by flow cytometry is mostly based on marker proteins which are limited by the number of fluorescence channels while immunohistochemistry can only evaluate a number of populations at a certain time. To circumvent these limitations, the present study used a transcriptomic deconvolution algorithm to enumerate immune cell abundances from a previous aneurysm gene expression dataset. The resolving power is the benefit of the algorithm, which has been validated with significant clinical implications [12-14].
The work presented here estimated the relative proportions of major immune cell subtypes within unruptured and ruptured IA by deconvoluting bulk gene expression data from these aneurysm walls. The findings show increased abundance of certain lymphoid immune cells in unruptured aneurysms and identified novel immune populations not previously characterized in this vascular phenotype.
Materials and Methods:
A previously published dataset consisting of IA gene expression profiles was used for this study [15]. The dataset consists of bulk tissue RNA-sequencing data of tissue from 21 ruptured and 21 unruptured IA. The data was generated using the Illumina HiSeq 2500 platform and was downloaded from the Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo) under accession GSE122897. The raw reads were converted to TPM (transcripts per million) space prior to input into the algorithm.
CIBERSORTx, an established machine-learning deconvolution algorithm, was implemented to provide an estimation of the relative abundances of immune cells from the original CCM lesions [16]. The algorithm accurately quantifies the relative fractions of distinct cell types within a complex gene expression mixture given cell type-specific gene signatures. For this, a validated microarray-derived signature matrix for distinguishing 22 human hematopoietic cell subsets was used to deconvolute the lesion tissue gene expression profiles [17]. The CIBERSORTx algorithm was run with bulk-mode batch correction and 500 permutations in relative mode. Quantile normalization was not implemented as dataset was generated from RNA-sequencing. Deconvoluted samples were deemed significant if CIBERSORTx P-value < 0.05, which represents the significance of the deconvolution results across all cell subsets for goodness-of-fit [16].
The data output was downloaded and analyzed with R programming language. Normality was not assumed and no values were excluded, thus the non-parametric Mann Whitney U test was used for comparisons. P-value < 0.05 was deemed significant.
Results:
After RNA deconvolution for immune cell subset identification, all 42 samples were significant at the criteria for CIBERSORTx P-value < 0.05 and were deemed suitable for further analysis. Comparing unruptured to ruptured IA showed increased plasma cells, CD8+ T cells, and activated natural killer cells in unruptured IA (shown in Fig. 1-3).
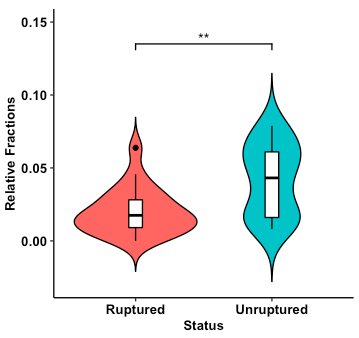
Fig. 1. Violin plot of relative abundances of plasma cells estimated from ruptured and unruptured intracranial aneurysms. Overlaid boxplot represents upper and lower quartiles, with black notch depicting the median. Whiskers depict 1.5 times IQR. **P < 0.01.
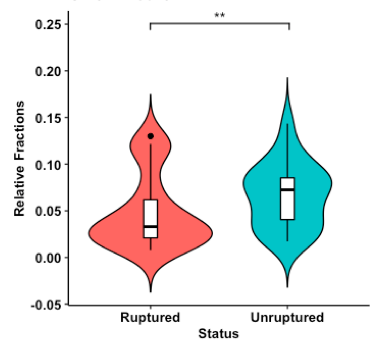
Fig. 2. Violin plot of relative abundances of CD8+ T cells estimated from ruptured and unruptured intracranial aneurysms. Overlaid boxplot represents upper and lower quartiles, with black notch depicting the median. Whiskers depict 1.5 times IQR. **P < 0.01.
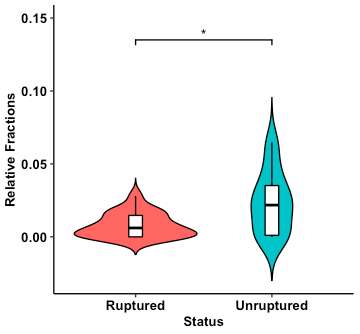
Fig. 3. Violin plot of relative abundances of activated natural killer cells estimated from ruptured and unruptured intracranial aneurysms. Overlaid boxplot represents upper and lower quartiles, with black notch depicting the median. Whiskers depict 1.5 times IQR. *P < 0.05.
Discussion/Conclusion:
The present study found certain immune cell subtypes are present at increased concentrations in unruptured IA compared to ruptured IA walls. Importantly, these findings elucidate specific immune subsets involved, such as CD8+ T cells. T cells have already been associated with IA as the second most abundant infiltrates [18-19]. More importantly, a recent study found decreased peripheral CD8+ T cells in the acute phase of subarachnoid hemorrhage compared to controls [20]. However, the mechanism behind this deregulation has not been examined. Plasma cells are terminally differentiated B-lymphocytes that develop a characteristic morphology and robustly produce immunoglobulins. The increased presence of these cells in unruptured IA walls suggests that the inflammatory reaction is initiated by a humoral immune response. Previous studies have described sporadic B-lymphocytes in unruptured aneurysm tissue [18]. Prior immunohistochemistry examinations have also recorded the presence of IgG and IgM immunoglobulins in the majority of the investigated aneurysm walls [18, 21]. In addition, these results point toward a role for natural killer cells in the pathogenesis of IA. With regards to aneurysms, natural killer cells have been found in high percentages in abdominal aortic aneurysms with little data describing their involvement or presence in unruptured IA [22]. It is possible these cells exert a cytotoxic effect and contribute to endothelial injury within IA walls prior to rupture. However, since there was a delay of >48 hours between subarachnoid hemorrhage and clipping in half the patients analyzed, the overexpression of immune response gene profiles may be an artifact of an inflammatory reaction in response to the rupture instead of a contribution to the rupture.
The limitations of this study include the in-silico approach to deconvolute gene expression data from bulk tissue sample. The data processing does not account for the spatial relationship of tissue and cytoarchitecture. Nonetheless, based on the hypothesis-generating data presented here, further studies are warranted to elucidate the exact mechanisms and interactions of these immune subsets in IA rupture.
Statements:
Statement of Ethics:
This article does not contain any studies with human participants performed by the author.
Disclosure Statement:
The authors have no conflicts of interest to declare.
Funding Sources:
Not applicable. No funding was received for this study.
Author Contributions